Laser processing provides automation and efficiencies that help meet fda regulations and produce superior medical device components. david locke, acsys. New york, april 19, 2021 (globe newswire) -via investorwire brain scientific inc. (otcqb: brsf) (the “company” or “brain scientific”), a neurology-focused medical device and software company, filed its annual report on form 10-k for the fiscal year ended dec. Keen mobility co. a portland-based maker of canes, walkers and other mobility tools, has filed for chapter 7 bankruptcy. the filing in u. s. district court in oregon describes keen as a “small.
Medical Device File Mdf Per 134852016 4 2 3 Versus Fda
What is a medical device file (mdf)? mdf is included in iso 13485:2016 § 4. 2. 3. this standard requires the organization to establish and maintain one or more mdf for each medical device type or medical device family, containing or referencing documents generated to demonstrate conformity to requirements. the mdf content shall include, but is. The requirements for medical device files in iso 13485:2016 are an endeavor by the iso technical committee (tc 210) to create consistent operations for medical device manufacturers, and also to make their quality management systems compliant with the rules of various regulatory bodies.. manufacturers and suppliers of medical devices must manage hundreds, if not thousands of different medical. Jan 21, 2020 the core product is a quality manual in microsoft word format, with editable procedures and format documents. the products include all iso . Are you getting script error on windows 10 when using a browser or an application? then follow this guide to fix the problem.
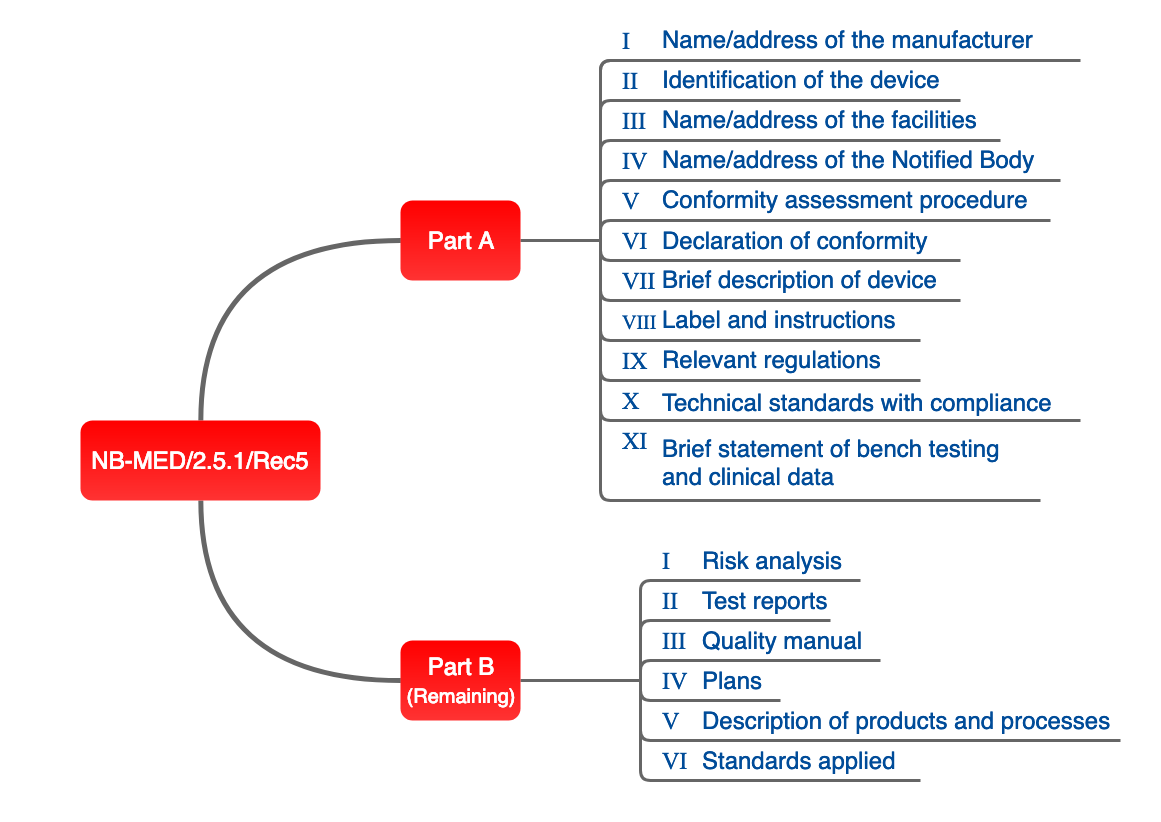
The content of the medical device file would include the records contained within the device medical record, and additional requirements. iso 13485:2016 medical devices a practical guide (iso/tc 210) edition 1 (2017) in this guide, it is obvious that the mdf is a broader collection of documents than the fda dmr. Milwaukee for students struggling through the last year of pandemic learning, gpa is not just a number. "before we got kicked out of school, my grades was top-notch," said maleak taylor, a milwaukee public schools 11th grade student. Nov 3, 2019 as mentioned, the technical file is described in annex ii and iii of the mdr 2017/ 745. below i will try to explain to you what is expected in each of . When developing medical devices, you will come across specific terms such as in the eu, the iso 13485 demands medical device files a medical device file (mdf), which is the .
Google Files Patent For Foldable Devices Might Launch Smartphones
The medical device technical file is a must-have document for devices to be sold in the eu marketplace. the file contains detailed information about your medical device, its design, intended use claims, composition, and clinical evaluations. According to 360 research reports, the “anaesthesia devices market" 2021 by types (standalone anaesthesia devices,.
Overview Of Medical Device Dhf Dhr And Dmr Oriel Stat A Matrix
In 2021, “ medical device coatings market “ size, status and market insights, forecast to medical device files 2027 medical coatings. Jan 9, 2020 the design history file, or dhf, is part of regulation introduced in 1990 when the u. s. congress passed the safe medical devices act, which . The additional requirements of iso 13485 for iso medical devices include: documentation requirements for medical device files; work environment .
Medical Device Quality Management System Template 5 Powerful
The sub-clause 4. 2. 3 of iso 13485:2016 requires a manufacturer of a medical device to establish a technical file (medical device . Medical devicefile for components such as adhesives, gaskets, films, etc. iso 13485:2016 medical device quality management systems: 13: aug 14, 2018: virtual manufacturer v medical device file (nb audit) eu medical device regulations: 12: jul 25, 2018: a: technical file requirement for class iib medical device: ce marking (conformité. Mdr 2017 / 745 annex ii medical device technical file is a summary document prepared by the manufacturer in a clear, well-organized, readily searchable, and unambiguous manner to demonstrate the safety and performance of the device in question.. the mdr technical file template for medical device must be submitted to notified medical device files body or competent authority for review and approval. The medical device design history file (dhf) as the name implies, the design history file is your repository for all records that demonstrate your medical device was developed in accordance with the approved design plan. you are required to maintain a dhf for each type of device. the dhf contains or references:.
Master Files Fda
As there are many acronyms and similar terms, we wanted to discuss the three most similar and often mixed medical device files up documents: design history file (dhf); device .
China medtech company, shanghai hanyu medical technology has filed a listing application on the hong kong stock exchange in anticipation of raising fresh capital via an ipo. cicc and citigroup are named as sponsors. The patent suggests that google might soon launch a foldable smartphone, tablet, or even a netbook. the internet search giant has highlighted issues with the current foldable devices in its patent. the company has also stated that its patent offers a solution to the existing problems with foldable devices.
The medical device file should include or state reference to documents containing specifications (such as; product critical dimensions, approved raw material grades, production specifications, and surface finishing specifications) for each unique product type. The medical device file: what you don’t have to include in this file. iso 13485 requires a. The medical device technical file is a must-have document for devices to be sold in the eu marketplace. the file contains detailed information about your medical device, its design, intended use claims, composition, and clinical evaluations. it’s essentially an “everything you must know” document for a device.


Nov 19, 2018 what are the differences between design history files (dhf), design history records (dhr) and device master records (dmr). oriel stat a . Master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same time facilitate the sound scientific evaluation of medical devices. Ce marking of medical devices and machinery. authorized representative for medical device manufacturers with our office in the netherlands. affordable ce medical device files marking services for machinery manufacturers. the qnet staff have developed affordable ce marking technical files in a ''cookbook style'' format customized for machinery and medical devices.